Introduction
The captain of a spacecraft approaching a new planetary system calls his exobiology officer and asks him to determine whether any of the planets bear life. With a remote sensing device the officer is soon able to provide a confident yes or no answer.
Although this is a familiar scenario of science fiction, is such a device really feasible? Let us consider the possibilities.
I have previously suggested that the chemical composition of a planet's atmosphere could be used as an indicator of the presence or absence of life using observations with a telescope from afar.* (* Nature, 1965, vol. 207, p. 568; Icarus, 1967, vol. 7, p. 149.) If the biology on a planet, such as the Earth, merely adapted itself to the chemical composition of its atmosphere without changing it, then the idea would not work. However, if the atmosphere is actively affected and altered by life on the planet, then its composition could provide definite clues about the presence of life. If fact [sic(?); should "If fact" be "In fact"?], with Lynn Margulis, I have gone further and proposed an hypothesis that the Earth's biosphere (atmosphere, oceans, and crust plus plant and animal life) is a system which is able to adjust the environment to be an optimum for its needs. In this, the Gaia hypothesis, this optimization applies not only to chemical abundances in the atmosphere but also to the climate, the surface acidity and other important planetary properties.
Defining the Problem
The problem lies not so much in the design of the telescope as in the search procedure and in the interpretation of the information it gathers. We are so accustomed to recognizing terrestrial life by instinctive processes, that the basis of this recognition is rarely analyzed or questioned. To recognize alien life at a distance through a telescope we need something more than our internal terrestrial life recognition program. The science of heat transfer (thermodynamics) provides clues and guidance.
First, there is a requirement known as the second law of thermodynamics that systems run irreversibly "downhill" toward a state of equilibrium. Thus, mixing a glass of hot water with a glass of cold water results in a warm water mixture. This process is irreversible in the sense that the warm water mixture cannot be divided again into a glass of hot water and one of cold. However, life processes can go against this irreversible downhill trend by taking in substances or free energy from the environment.
If the life of a planet is chemically based and driven by a star's radiation, gaseous products may diffuse into the atmosphere and by their presence cause a departure from the equilibrium state. Thus, the atmosphere becomes a part of the system.
 Second, it is intuitive to link life as a system with those processes which occur when the change of free energy is larger than a certain value. Such a limit is well recognized in studies of fluids as the Reynolds number which marks the onset of vorticity or whirlpool-like formations. Like life, vortices establish characteristic recognizable forms with life-spans and histories, and when they form they confer a new sort of dynamic stability upon their environment. On the larger scale in the atmosphere, such vorticity sometimes evolves to the intricate structure of a hurricane and a recognizability great enough to qualify for personal naming.
Second, it is intuitive to link life as a system with those processes which occur when the change of free energy is larger than a certain value. Such a limit is well recognized in studies of fluids as the Reynolds number which marks the onset of vorticity or whirlpool-like formations. Like life, vortices establish characteristic recognizable forms with life-spans and histories, and when they form they confer a new sort of dynamic stability upon their environment. On the larger scale in the atmosphere, such vorticity sometimes evolves to the intricate structure of a hurricane and a recognizability great enough to qualify for personal naming.
The importance of this development for the problem of life detection is that it provides a formal distinction between irreversible processes and life-like processes.
For example, within the appropriate range of something equivalent to a Reynolds number a planet might be expected to bear life whereas outside that range it would not. Maelstroms do not develop in duck ponds, neither could life start or continue in an environment which was otherwise ideal, but which lacked a free energy rate of change of sufficient magnitude. Such may well be the conditions deep on Jupiter, warm, damp, with all of the chemical components but lacking free energy. The possibility that convection may convey sufficient reactive molecular species from the upper atmosphere leaves open the question of the possibility of life on Jupiter.
Third, from the standpoint of probability, living systems possess a molecular configuration of extreme improbability when viewed against the highly probable equilibrium background condition.
Designing the Experiment
Turning now to the experiment, let us assume that the planet can be seen clearly enough through the telescope to provide the following information:
(1) Surface temperatures to within ± 20 degrees (kelvin).
(2) The presence of bulk water and ice and its surface distribution.
(3) The atmospheric abundance of chemically active gases down to one part in 100 million.
(4) The distribution of temperature, pressure and chemical composition with altitude and with surface coordinates.
The resolution of this last measurement should be sufficient to distinguish between at least the major mixing zones of the planet. For the Earth this would include the northern and southern hemispheres, the troposphere, the stratosphere and the upper atmospheric regions.
The methods are already at hand to gather most of these quantities for a planet such as Mars from the distance of the Earth. The instrumental methods would be spectroscopic and range in wavelength from the short ultra-violet to the long infra-red. To gather the information requires no visionary instrumental development, merely some improvements in current hardware.
This approach to life detection assumes that the life is chemically based, that it is situated at the planetary surface and that it uses the atmosphere as a transport medium and also as a storage space for raw materials. Even if it is possible to distinguish any departure from equilibrium associated with life, it can only apply if the life in question has modified that part of the planet which is observable. If life was too sparce ["sparce" is spelled "sparse" in the U.S.] or existed only below the surface of the planet, neither it nor its effects might be visible. There are reasons for believing, however, that once life is initiated on a planet it can only persist if it is able to control the planetary environment. This ability may be an important evolutionary step in the early stages of life. The early growth of life on a planet will certainly change the surface and atmospheric composition and such changes could easily alter the radiation environment to a state unfavorable to life. A failure to learn to control the planetary conditions at this early stage could be fatal. Life as a going concern is likely to be intense and to have profoundly modified the physical and chemical environment of the planet.
In developing the Gaia hypothesis, Margulis and I postulated that the biosphere has properties which enable it to control at least the following variables:
(1) Surface temperatures.
(2) Atmospheric composition.
(3) Surface and ocean acidity.
(4) Ocean composition.
(5) Materials balance.
In this hypothesis the biosphere is, like its constituent plant and animal parts, seen as a complex automatic control system with the capacity to adjust its environmental balance to its advantage. At present Gaia is only a hypothesis but if we can establish the reality of a tendency toward stability for the Earth then it may also be a general characteristic of biospheres. If it is, their recognition from afar will be much easier.
Experimental Evidence
Now let us consider the information available about the planets Jupiter, Mars, Earth and Venus in this context. To keep within the constraints of a realistic experiment the information about the Earth is restricted to that which could be gathered from the distance of Mars.
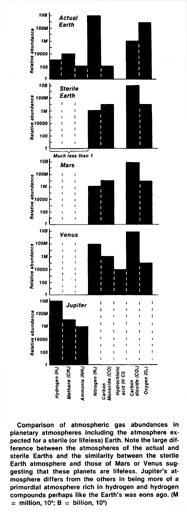 The figure shows the amount of chemically reactive gases in the atmospheres of the four planets; also included are the gases of a lifeless or sterile Earth. The gas concentrations are expressed in logarithmic units so that the minor constituents can be compared as well as the principal gases.
The figure shows the amount of chemically reactive gases in the atmospheres of the four planets; also included are the gases of a lifeless or sterile Earth. The gas concentrations are expressed in logarithmic units so that the minor constituents can be compared as well as the principal gases.
The outstanding difference between the (actual) Earth and the other planets is the simultaneous presence of molecular hydrogen, methane and ammonia with that of oxygen. If the gases of the atmosphere were allowed to react among themselves and with the crustal rocks and ocean the final equilibrium would be that marked Sterile Earth. One indication of life is the presence of these gases (hydrogen, methane and ammonia) in the actual Earth's atmosphere in amounts which are more than a trillion trillion (1024) times greater than they would be in a sterile Earth's atmosphere.
When considered singly and in isolation, plausible inorganic origins can be assigned to most of these gases but when the ensemble is considered together with oxygen we have something so unusual as to be beyond any credible inorganic chemistry. Any lingering doubts are dispelled by a consideration of the quantities of these gases. To sustain the present concentration of methane requires a billion tons a year and, also equally significant, 4 billion tons a year of oxygen. This much methane would require that all of the carbon in Earth's crust was turned over every 100 million years. This is normal for a biosphere but incredible as an accident. The discovery of almost any biochemical in neutral or reducing environment, even a protein, would not be conclusive evidence of life. The discovery of even a simple reduced carbon compound such as methane in sufficient quantity in an oxydizing atmosphere is, however, strong evidence for a dynamic system which is making it.
|
Atmospheric Recycling
The atmosphere is a huge mixing machine. It contains about 6 million billion (6 x 1015) tons of air. The earth's human population breathes about 25 billion tons of air per year so that humans breathe 4 parts per million of the atmosphere per year. Put another way, this means that for each breath a person takes, 4 parts per million were breathed by some human during the previous year. This amount could be more or less if mixing is incomplete. This is a measure of human air recycling which will increase in proportion to the population.
There are about 20 billion trillion (20 x 1021) atoms in a breath of air. Thus, for each breath one takes there are more than a million billion (1015) atoms in that breath which were exhaled by other humans during the past year and almost a billion atoms that were breathed by
any adult human that ever lived.
— Ed.
|
Other Biospheres
Even though the discovery of life on the Earth through the recognition of its biosphere may not be difficult, it does not follow that this is a generality. We cannot yet test the method against other planetary biospheres, but we can ask if it could have detected past biospheres on the Earth. We know that life has existed on the Earth for 3 billion years and although the view is poor from this distance in time some of the larger features can be seen. When life began there was probably more hydrogen and methane with less oxygen while during the evolution of the Earth's biosphere there has been a shift in the amounts.
If we assume that soon after the start of life a biosphere with automatic control is established; that the planet is developed to the limit of its resources and of the energy it receives from its star; that it is maintained at optimum conditions for whatever is the current biosphere; then the
amounts of these gases indicate the status of life processes on the planet as viewed from afar.
Truly Alien Biospheres
What of truly alien biospheres? The universe appears to be littered with the spare parts from which our current chemical life form is composed. From this fact and from our knowledge of properties of the elements it seems unlikely that other, for example, silicon based life forms are likely to have evolved. On the other hand, we have ample experience with the possibility of life based on electronic and mechanical contrivances; a biosphere in this form would evolve presumably from a chemical one, perhaps starting in cooperation with it. Could we recognize such a biosphere? If it is a property of biospheres to optimize their use of raw material and free energy to control the planetary surface conditions at those most favorable for survival, then this form of biosphere or an alien chemical one should be recognizable. Except by a purposeful act of camouflage any life system will reveal its presence through the chemical disequilibria caused by its contrivances.
Compounds now present in the Earth's atmosphere which might be seen by infra-red analysis from afar are the chloro-fluoro-carbons — the highly contrived chemicals made for use as refrigerants and aerosol propellants. They could not have arisen accidentally by inorganic chemistry and their presence would be indicative, for example, of a biological life which had developed a chemical industry.
Much of this discussion has been concerned with the properties of planetary atmospheres. This is partly because more information is likely to be available about planetary atmospheres than about their surfaces and partly because the physical chemistry of low pressure gas mixture is considerably easier to consider than mixtures including also solids and liquids. But a view of a planet does provide information on such surface features as clouds, oceans, reflectivity, radiation and the distribution of temperatures and chemical substances. These all can add weight to the sum of evidence concerning the presence or absence of life.
Conclusions
This work started 15 years ago, motivated by the need to design a planetary life detection system which was not geocentric and could be used, for example, for Mars. Applied to the Earth, which is the only planet we know with life, there is a great difference in its atmospheric gases from those expected in the absence of life (sterile Earth). By contrast, what we know about the other planets shows no sign of a significant departure from that expected for a sterile biosphere.
If future planetary explorations confirm the absence of life on the other planets of our solar system, then the remote detection of life by telescope might be established as a method, at least for planets like the Earth. It may even be a general method of planetary life detection and the remote sensing device of science-fiction could become a reality.
It may be some time before we can try these tests for life on the planets of other stars but in the meanwhile what started as an exercise in life beyond the Earth could become an expedition to discover the largest living creature on Earth, Gaia.
This article is a popularized condensation based on "Thermodynamics and the Recognition of Alien Biospheres" by James E. Lovelock, ![[NAAPO Logo]](../../Images/NAAPOsm.jpg)
![[NAAPO Logo]](../../Images/NAAPOsm.jpg)