Isaac Newton demonstrated that a glass prism could separate white light into a rainbow of colors from violet through blue, green and yellow to red. These colors represent different wavelengths of light. Violet wavelengths are shorter while red wavelengths are longer. Newton's rainbow is called a spectrum which, for visible light, extends from a wavelength of 400 nanometers at the violet end to 700 nanometers at the red end. This is the visible spectrum to which the human eye is sensitive. A 2-to-1 difference in wavelength is one octave. The ratio of 700 to 400 is less than 2 to 1 so the visible spectrum is less than one octave wide. By way of comparison, the difference in pitch between the musical notes of middle and high C is one octave, pitch being to sound what color is to light.
Although light is an electromagnetic wave, it may also be referred to as a ray. The term wave calls attention to its wavelike nature while the word ray emphazies the path of propagation which is a straight line in a uniform medium.
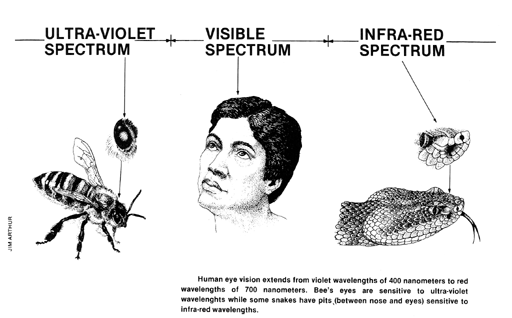
Wavelengths shorter than violet (less than 400 nanometers) are called ultra-violet and those longer than red (longer than 700 nanometers) are called infra-red. Bee's eyes are sensitive to ultra-violet wavelengths almost as short as 300 nanometers and some snakes have receptors or pits between their nose and eyes which are sensitive to the infra-red wavelengths.
William Herschel, the musician turned astronomer, was the discoverer of infra-red radiation. He had tried glasses of various colors to darken the sun while observing it with his telescopes and found that although red glasses reduced the light effectively they allowed heating of the eye with attendant irritation. He found that dark green glasses or smoked glasses worked much better, reducing both the light and heat. To learn more about the heating and illuminating power of the
sun's rays he contrived an experiment. Using a prism to spread out the sun's light into its spectrum or colors he placed a thermometer successively in the regions of different colors. He noted that the thermometer reading was lowest when in the violet region, higher in the green and even higher in the red. He discovered further that if the thermometer was moved just out of the red end of the spectrum so that it was not illuminated by the sun's light, its temperature went still higher. There were obviously invisible rays carrying the heat, rays which were refracted by the prism and akin to light.
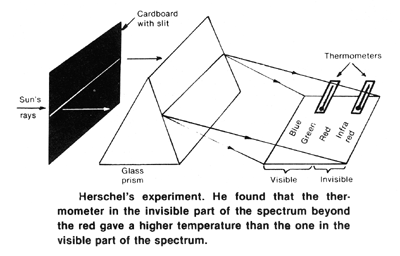 In a series of papers before the Royal Society in London in 1800, Herschel reported on his experiments and called "these invisible rays which produce no illumination but create a sensation of heat, calorific rays or radiant heat." To describe the light rays which produce visible illumination but little heat he used the contrasting term "colorific rays." In further experiments Herschel found that the maximum illumination occured in the yellow-green region in the middle of the "colorific" spectrum (550 nanometers). For calorific and colorific rays we now use the terms infra-red and visible rays.
In a series of papers before the Royal Society in London in 1800, Herschel reported on his experiments and called "these invisible rays which produce no illumination but create a sensation of heat, calorific rays or radiant heat." To describe the light rays which produce visible illumination but little heat he used the contrasting term "colorific rays." In further experiments Herschel found that the maximum illumination occured in the yellow-green region in the middle of the "colorific" spectrum (550 nanometers). For calorific and colorific rays we now use the terms infra-red and visible rays.
A pavement or brick wall baked by a late afternoon sun can, in the darkness of evening, warm one's body from a distance. The heat is conveyed by invisible infra-red rays. Our skin is sensitive to infra-red rays but not to light unaccompanied by heat or infra-red rays. Conversely, our eyes are sensitive to visible light but hardly at all to infra-red rays.
If our eyes were sensitive to infra-red waves instead of light a warm room would appear brighter than a cold one and the inside of a refrigerator would be very dark indeed. A person wearing a white shirt and black pants would appear to have a dark shirt and bright pants. At some infra-red wavelengths (10 micrometers) the sky would appear bright by both day and night while at other wavelengths (2 micrometers) it would appear dark.
In 1801, only a year after Herschel had discovered invisible heat rays beyond the red end of the visible spectrum, John Wilhelm Ritter of Silesia found that invisible rays from the sun beyond the violet induced changes in chemical compounds. Accordingly, these rays were called "chemical" rays. We now use the term "ultra-violet."
Although our eyes are not sensitive to the ultra-violet rays from the sun (between 300 and 400 nanometers), our skin reacts to them by acquiring a tan. It is fortunate that the atmosphere is opaque to ultra-violet rays shorter than about 300 nanometers. If these even more chemically active rays were to penetrate to the earth's surface they would produce biological and ecological effects which could upset the environmental balance. The blocking of rays just short of 300 nanometers is due to absorption by the ozone of the upper atmosphere. The ozone molecules, consisting of three oxygen atoms, are formed from the normal two-atom molecules of oxygen by the ultra-violet rays of the sun.
Mountain climbers at high altitudes quickly acquire a shallow tan from the sun. This results from shorter ultra-violet waves than can reach sea level. The shallow, high-altitude tan doesn't last long. At sea level the tan is due to the longer ultra-violet waves which penetrate the skin more deeply and impart a longer-lasting tan.
Ordinary photographic film responds to ultra-violet while special red sensitive film can be used for infra-red radiation to wavelengths as long as 1000 nanometers. Wavelengths between 300 and 1000 nanometers penetrate the Earth's atmosphere. Throughout this range of wavelengths, called the optical window, ground-based astronomical observations are possible.
Wavelengths longer than 1000 nanometers (1 micrometer) require special thermal detection devices and for wavelengths as long as 1000 micrometers (1 millimeter) the infra-red blends into the short radio wavelengths. Although ground-based astronomical observations can be made through several narrow windows in the 1 to 35 micrometer region, astronomical observations at longer wavelengths to the shortest radio wavelengths are best made from above the atmosphere. Radio waves to lengths of many meters penetrate to the ground through what is called the radio window. However, long radio wavelengths are cut off, not by the atmosphere, but by a charged particle layer above it called the ionosphere.
The ultra-violet spectrum extends to about 10 nanometers where it blends into the long wavelengths of the x-ray part of the spectrum. The Earth's atmosphere is opaque to wavelengths less than 300 nanometers.
Ultra-violet, infra-red, light, x-rays, gamma rays and radio waves are all electromagnetic, extending over more than 50 octaves from gamma rays with wavelengths so short it takes billions of them to stretch across the period at the end of this sentence, to radio waves many kilometers in length. With the advent of telescopes in space above the Earth's atmosphere and ionosphere, this enormous range of wavelengths is now becoming accessible for astronomical observations. In coming "ABCs of Space" I will discuss the "new astronomies" from gamma rays to long radio waves which observations from space are making possible.
This discussion of infra-red and ultra-violet is adapted from a few pages of my new book, "Our Cosmic Universe," Cygnus-Quasar Books, 1980, copyright © John D. Kraus. The Herschel experiment figure and the optical window figure are also from the book.

![[NAAPO Logo]](../../Images/NAAPOsm.jpg)
![[NAAPO Logo]](../../Images/NAAPOsm.jpg)